This article explores the history, features, significance, and limitations of the Rutherford model, highlighting why it continues to be a cornerstone in the study of atomic structure.
Table of Contents
Background Context
At the turn of the 20th century, scientists sought to understand the true nature of atoms—the basic building blocks of matter. J.J. Thomson’s plum pudding model suggested that the atom was a positively charged “pudding” embedded with negatively charged electrons, much like raisins in a cake. While this model explained some phenomena, it failed to account for experimental evidence related to atomic behavior under particle bombardment.
Rutherford, a physicist with a keen interest in radioactivity and particle interactions, questioned the accuracy of the prevailing atomic model. He believed a more refined structure existed, which would eventually be revealed through experimentation.
The Gold Foil Experiment
In 1909, Ernest Rutherford, along with Hans Geiger and Ernest Marsden, conducted the now-famous gold foil experiment. They directed a stream of alpha particles (helium nuclei) at an extremely thin sheet of gold foil, expecting the particles to pass through with minimal deflection based on Thomson’s model. Most alpha particles did pass through as expected, but a small fraction were deflected at large angles, and some even bounced back.
This surprising result led Rutherford to conclude that the atom’s mass and positive charge were concentrated in a small, dense core—the nucleus—while the rest of the atom was mostly empty space. He famously remarked that it was “as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Key Features of the Rutherford Model
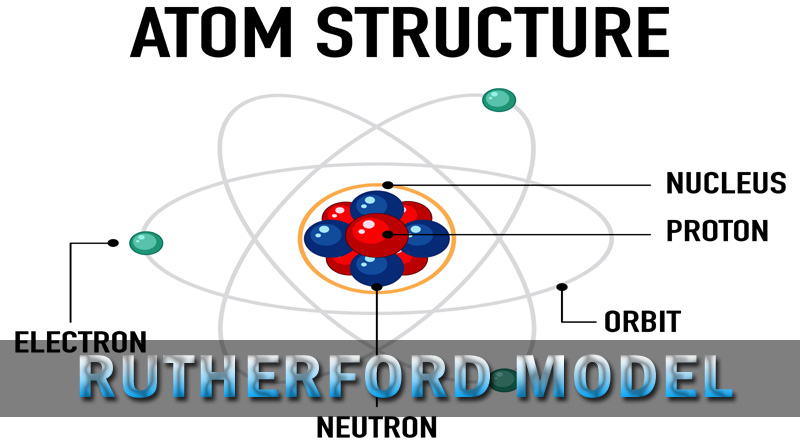
- The atom contains a small, dense, positively charged nucleus at its center.
- Electrons revolve around the nucleus in circular orbits, similar to planets orbiting the sun.
- Most of the atom’s volume is empty space, explaining the penetration of most alpha particles in the experiment.
- The nucleus contains nearly all of the atom’s mass, while electrons contribute little to the overall mass.
These features dramatically altered the scientific perception of atomic structure and laid the groundwork for future models.
Advantages of the Rutherford Model
The Rutherford model was revolutionary because it provided the first clear evidence that atoms are not uniform spheres. Its advantages include:
- It explained the large-angle scattering of alpha particles observed in the gold foil experiment.
- It introduced the concept of a central nucleus, which is fundamental to nuclear physics and chemistry.
- The model inspired further research, leading to the discovery of protons, neutrons, and quantum mechanical models of the atom.
Limitations of the Rutherford Model
Despite its significance, the Rutherford model had several limitations:
- It could not explain why negatively charged electrons, revolving around the nucleus, did not spiral into the nucleus due to electrostatic attraction.
- It failed to account for the discrete spectral lines observed in atomic emission spectra.
- The model could not explain atomic stability or energy quantization, issues later resolved by Niels Bohr’s atomic model.
Significance in Modern Science
The impact of the Rutherford model on modern science cannot be overstated. By identifying the nucleus as the atom’s core, Rutherford laid the foundation for nuclear physics, enabling breakthroughs such as nuclear energy, medical imaging, and particle physics experiments. The model’s introduction of empty space within atoms helped shape the development of quantum mechanics, which further refined our understanding of atomic behavior.
Even though the model has been superseded by more sophisticated theories, it remains an essential teaching tool in physics and chemistry education, demonstrating the scientific method and the evolution of scientific knowledge.
Comparison with Other Atomic Models
To fully appreciate the Rutherford model, it is helpful to compare it with other atomic theories:
- Thomson’s Plum Pudding Model: Depicted the atom as a positively charged sphere with embedded electrons. Rutherford’s findings disproved this by showing that positive charge is concentrated in a nucleus.
- Bohr Model: Improved upon Rutherford’s model by introducing quantized electron orbits to explain atomic spectra and stability.
- Quantum Mechanical Model: Modern atomic theory that uses probability distributions to describe electron positions, building on the foundation laid by Rutherford’s discoveries.
Legacy of Rutherford’s Discoveries
Rutherford’s contributions extended beyond the Rutherford model. His discovery of the proton, along with his research in nuclear transmutation, earned him recognition as the father of nuclear physics. The model’s introduction of a dense nucleus remains a cornerstone concept in atomic and nuclear research.
The Rutherford model also illustrates the dynamic nature of scientific knowledge—how theories evolve as new evidence emerges. Its role in inspiring further research demonstrates how even “incomplete” models can propel science forward.
Applications and Educational Value
While modern physics has moved beyond the Rutherford model, it continues to play an important role in education and introductory physics courses. Teachers use it to explain:
- The experimental basis of atomic theory.
- The importance of observation and inference in scientific discovery.
- The historical progression of atomic models, from Dalton to quantum mechanics.
Its simplicity makes it a powerful teaching tool for understanding basic concepts before introducing more advanced quantum mechanical ideas.
Conclusion
The Rutherford model was a milestone in the study of atomic structure. By demonstrating the existence of a dense nucleus and reshaping our view of the atom, Rutherford’s work set the stage for groundbreaking discoveries in quantum mechanics, nuclear physics, and chemistry. Though later replaced by more accurate models, its historical and educational significance remains unmatched. The Rutherford model continues to remind us of the power of experimentation, critical thinking, and the ever-evolving nature of scientific knowledge.
Frequently Asked Questions About the Rutherford Model
What is the Rutherford model?
The Rutherford model is an early atomic model that describes atoms as having a small, dense, positively charged nucleus with electrons orbiting around it.
Who proposed the Rutherford model?
Ernest Rutherford proposed the Rutherford model in 1911 after his gold foil experiment revealed that atoms have a concentrated nucleus.
What experiment led to the Rutherford model?
The gold foil experiment, conducted by Rutherford, Geiger, and Marsden, provided evidence for the nucleus and inspired the Rutherford model.
What are the key features of the Rutherford model?
Key features include a dense nucleus containing most of the atom’s mass, electrons revolving around the nucleus, and the atom being mostly empty space.
Why was the Rutherford model significant?
It revolutionized atomic theory by disproving the plum pudding model and paved the way for Bohr’s model and quantum mechanics.
What are the limitations of the Rutherford model?
It could not explain the stability of electrons in orbit or the discrete spectral lines observed in atomic emission spectra.
How did the Rutherford model improve on Thomson’s model?
It demonstrated that positive charge is concentrated in a nucleus, rather than being spread throughout the atom as Thomson proposed.
What replaced the Rutherford model?
Niels Bohr refined the concept by introducing quantized electron orbits, leading to the Bohr model of the atom.
Is the Rutherford model still taught today?
Yes, it remains an essential part of physics and chemistry education to illustrate the historical development of atomic theory.
What is the legacy of the Rutherford model?
The Rutherford model laid the foundation for nuclear physics and continues to be referenced as a critical step in understanding atomic structure.